85%
Cost Savings


The integration enabled seamless digital signatures for corporate credentials and SAFETM, streamlining approval and management of electronic lab notebooks and regulated documents. Previously, global shipping for signature collection caused delays and costs. The shift to digital signatures allows multiple users to sign instantly, improving efficiency and significantly reducing costs.
“George S. Rathbun, Director Of Engineering, Pfizer
Founded in 1849 and headquartered in New York, Pfizer is a leading global biopharmaceutical company focused on the discovery, development, and distribution of medicines and vaccines. Renowned for its innovation over 170 years, Pfizer leverages cutting-edge technologies to accelerate the creation of therapies that redefine healthcare. Its diverse portfolio is celebrated worldwide for effectively preventing and curing diseases.
The company is committed to sustainability, innovation, and leveraging digital solutions to enhance productivity and operational efficiency.
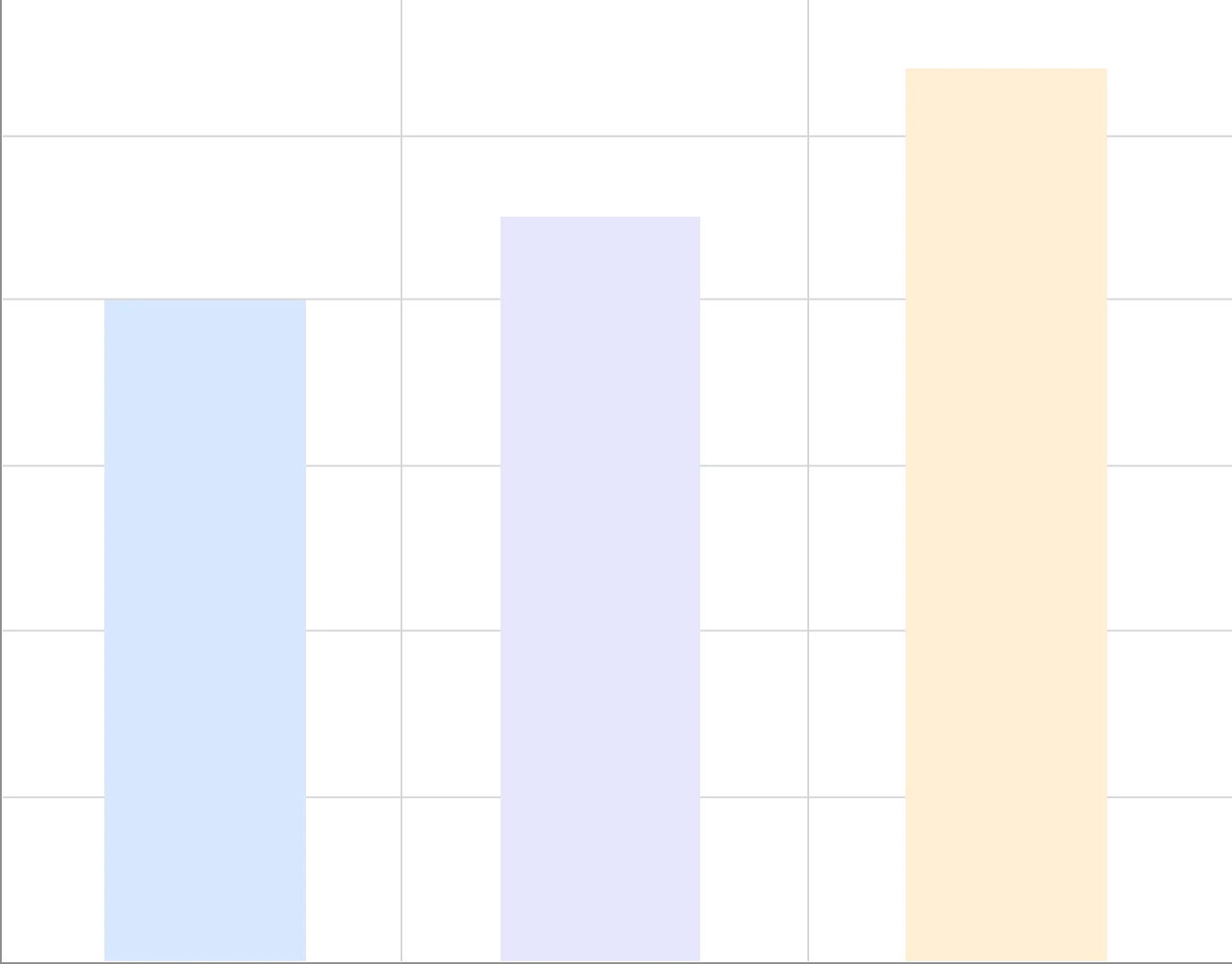
Cost Savings
Increase In Speed And Agility
In Drug Development
Improvement In
Documentation Efficiency
Pfizer’s extensive documentation processes relied on manual, paper-based workflows. Pfizer needed a scalable solution to address these challenges and streamline workflows. Experimental data recorded in paper lab notebooks caused inefficiencies, including:
 Prolonged approval
Prolonged approval
 Expensive printing
Expensive printing
 Poor lab efficiency
Poor lab efficiency
 Delayed compliance
Delayed compliance
MSB Docs integrated with Pfizer’s Chemistry Electronic Notebooks (CeN), enabling digital signing workflows. Experimental data is digitally signed by chemists and witnesses, with immediate archival for compliance and traceability.