
Pharmaceuticals: SOPs for Digital Compliance
In the highly regulated pharmaceuticals industry, ensuring compliance with digital processes is crucial for maintaining regulatory integrity.

How to Handle FDA Audits for Electronic Records Compliance
FDA audits are a crucial aspect of regulatory oversight for businesses handling electronic records, particularly those using Electronic Signature Cont

21 CFR Part 11 Compliance: Key Elements of a Validation Plan for 21 CFR Part 11 Systems
Ensuring compliance with 21 CFR Part 11 is a critical step for organizations in the pharmaceutical industry and other regulated sectors.

Pharma: Steps to Conduct a Self-Assessment for 21 CFR Part 11 Readiness
In the pharma industry, compliance with 21 CFR Part 11 is not optional—it’s a necessity. This regulation, established by the FDA, ensures that ele

Electronic Signature Contracts: Secure Storage & Retrieval
With businesses increasingly relying on Electronic Signature Contracts, securely storing and retrieving eSigned documents has become more important th

Pharma: Multi-Factor Authentication for 21 CFR Part 11 Compliance
Pharma companies operate in a highly regulated environment where data security and compliance are paramount. Ensuring compliance with 21 CFR Part 11 i

Electronic Signature Contracts: What FDA Requires
The shift from traditional wet signatures to electronic signatures (eSignatures) has transformed how businesses and regulated industries manage contra

e-Signature for Pharmaceutical: How Cloud-Based Digital Solutions Improve FDA Compliance
In the pharmaceutical industry, ensuring FDA compliance is non-negotiable. From clinical trials to vendor contracts, every process must adhere to stri

21 CFR Part 11 Compliance: Ensuring Data Integrity in FDA-Regulated Industries
In today’s digital era, maintaining data integrity is a top priority for FDA-regulated industries, particularly in pharmaceuticals, biotechnology, a

E-Signature for Pharmaceutical: Ensuring Compliance in Life Sciences
The life sciences industry operates within a highly regulated environment where compliance, security, and accuracy are paramount. From pharmaceutical

How to Achieve 21 CFR Part 11 Compliance in Your Organization
In today’s digital era, 21 CFR Part 11 compliance is essential for industries like pharmaceuticals, biotechnology, and healthcare. If your organizat

Pharmaceuticals: Why Pharma, Biotech, & Medical Devices Rely on 21 CFR Part 11 Compliance
In the highly regulated world of pharmaceuticals, biotechnology, and medical devices, ensuring compliance with stringent guidelines is not just a choi

21 CFR Part 11 Compliance: The Role of Audit Trails
In today’s digital era, regulatory compliance is a top priority for industries dealing with electronic records and signatures. If you work in pharma

Electronic Signature Contracts: Why Compliance Matters
In today’s digital landscape, businesses and organizations are constantly evolving their record-keeping processes. While paper-based records have be

21 CFR Part 11 Compliance: Key Requirements & Principles
In highly regulated industries like pharmaceuticals and life sciences, ensuring the security, integrity, and authenticity of electronic records and si

21 CFR Part 11 Compliance Guide: How MSB Docs Helps You Comply
In the pharmaceutical industry, where data authenticity and regulatory adherence are critical, compliance with 21 CFR Part 11 is not just an option �

Top 7 Reasons Regulated Industries Trust MSB Docs for 21 CFR Part 11 Compliance
In the highly regulated world of life sciences, compliance isn’t just about checking boxes—it’s about saving time, reducing risks, and ultimatel

How MSB Docs’ AI-Powered CLM and eSignature Solutions Mitigate Contract Risks and Errors
In today’s fast-paced business landscape, contracts have become the backbone of operations—whether dealing with employees, vendors, or clients. Bu

Conquer the Top 6 Quality & Compliance Oversights in Pharma
The success of any business in the pharmaceutical industry relies heavily on its ability to navigate the ever-changing regulations that are required f

Equip Your Drug Launch with Nitrosamine Regulations: Essential Guide
This guide is designed to help organizations understand and comply with the nitrosamine regulations related to marketing applications for new drug pro

Learn to Uncover Value from Clinical Trials in Australia
Australia is home to world-class medical research, and clinical trials are a major part of this. Clinical trials are federally regulated studies that

Unlock the Potential of Clinical Research: Why Decentralized Clinical Trials Are the Future
Decentralized clinical trials (DCTs) are a new approach to conducting clinical research that shifts the traditional model in significant ways.

Ready for Clinical Trial GCP Audits? Here’s What You Need to Know
A Good Clinical Practice (GCP) audit is an important part of clinical trials that ensures that the methods used and data collected are meeting certain

An In-depth Look at Integrity & Reliability in Bioequivalence Studies
Bioequivalence studies are an important process for ensuring that the pharmaceutical products produced by drug companies meet regulatory standards, su

FDA Announces New Reg. for Easy-to-Understand Medication Info for Patients
When it comes to taking medications, it’s important to understand what you’re consuming and the possible risks associated with doing so.

Take a Peek Into Common Critical Findings Found in Compliance Auditing
Compliance auditing is an important component of regulatory compliance. It involves a thorough review of procedures, processes, and records to ensure

Unlock Safety and Security with GVP: A Quick Guide
Good pharmacovigilance practices (GVPs) are procedures used to protect the safety of patients taking medications.

Uncovering FDA’s Nitrosamine Impurity Regulations for NDSRIs
NDSRI stands for Non-selective Dopamine Reuptake Inhibitor and is a type of medication used to treat depression and other mental health conditions.

Discover What the Drug Supply Chain Security Act (DSCSA) Means for You!
The Drug Supply Chain Security Act (DSCSA) is a law of the United States that was passed in 2013 to create a national system for the regulation and co

Data Integrity: Harness Its Power to Seamlessly Transition to Pharma 4.0
In the current digital age, data integrity has become increasingly important as organizations strive to transition into a more automated and efficient

Unlock the Benefits of FDA ISO 13485 and 21 CFR Part 820 Harmonization
The U.S. Food and Drug Administration (FDA) is the governing body that regulates medical device manufacturers.

Unlock Quality Improvement In Pharma W/ Smart Goals
A smart goal is an acronym for Specific, Measurable, Achievable, Relevant and Time-bound. It is an effective method used by many organisations to ensu

Uncover the UDI System: Why it Matters
The UDI System, short for Unique Device Identification System, is a system used to identify medical devices through the use of labels, barcodes, or ot

Solving Sustainability Challenges: Pharma Industry and Sustainability by Design
The pharmaceutical industry plays a critical role in the health and wellbeing of millions of people all over the world.
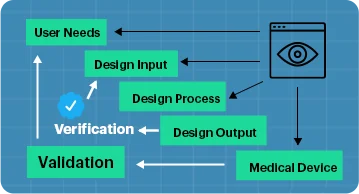
Gain Insight: Design Verification & Validation for Medical Devices
Design verification and design validation are processes used in the production of medical devices.

Overcome Critical Challenges with QbD: How to Implement QbD Successfully
Quality by Design (QbD) is an approach to drug development and manufacturing that focuses on optimizing the quality of the final product by understand

Discover the Pros & Cons of Pharmaceutical Contract Manufacturing
Pharmaceutical contract manufacturing helps businesses meet the increasingly complex demands of the industry.

Mastering Clinical Trials: Guarantee Quality with 4 Essential Phases
Clinical trials are essential for medical research and have proven to be a key element in advancing medical treatments and developing new drugs.
Unlock Pharmaceutical QA Mastery with 4 Easy Steps!
Today, the world of pharmaceuticals is becoming increasingly complex. With global health concerns rising to the forefront, pharmaceutical companies ar
Step-by-Step Guide to Starting a Pharmaceutical Company
Today, the world of pharmaceuticals is becoming increasingly complex. With global health concerns rising to the forefront, pharmaceutical companies ar

Unlock the Secrets of cGMP: 7 Experts Explain
Good Manufacturing Practice (GMP) is a set of guidelines, established by the World Health Organization (WHO), that focus on pharmaceutical manufacturi

Reduce Risk & Reap Benefits: Everything You Need to Know About Design Controls for Med Devices
Design Controls are an essential part of designing and developing any medical device. They help to ensure the safety, effectiveness and quality of a p

Dive Deeper Into Risk Management for Medical Device Startups
Risk management is an essential part of any business, and medical device startups are no exception. Without effective risk management, medical device

Unlock the Secrets of 21 CFR Part 11 Compliance for Medical Device Mfgs
21 CFR Part 11 is a set of regulations developed by the United States Food and Drug Administration (FDA) to enhance the protection of electronic recor

What Makes a Biopharmaceutical Startup Different from a Pharmaceutical One?
As one of the crucial stakeholders of health care, patients are involved in various aspects of treatment, leading to various controversial opinions ab

21 CFR Part 11 eSignature Compliance Checklist Here’s What You Need to Know About FDA Regulations (Updated)
Keeping sensitive data and private information secure is extremely important as well as complex

21 CFR Part 11 Compliance Checklist to Follow
21 CFR Part 11 outlines the FDA’s requirements for the integrity, quality, and compliance of digital documents and also signatures.

21 CFR Part 11 and Good Documentation Practices in Pharmaceutical Industries
It has been said that in the pharmaceutical industry, “If it isn’t documented, it didn’t happen.
How MSB Docs CRA Scan App Helps in Good Clinical Practice (GCP)
Advancement in the prevention, medication, and treatment of healthcare problems has only been possible because of the good clinical practices which

Why pharmaceutical industry loses Billions of dollars every year?
Pharma industry is one of the largest industries that is totally built on innovation, involving a huge investment in research and development (R&

Top 9 Digital Transformation Trends in Pharmaceutical Industry (Update 2021)
The pharmaceutical industry has seen massive growth in recent years, and it clearly shows the upward trend for 2021 as well. Technological advances ar

21 CFR Part 11 eSignature Compliance Checklist: What You Need to Know About FDA Regulations (Updated)
Every life sciences company that either sells or proposes to sell any kind of medical device has to follow the requirements under


