- Email us: sales@msbdocs.com

Glenmark Pharmaceuticals Ltd., founded in 1977, is a global, research-driven pharmaceutical company with a strong presence in India and operations in 80+ countries. It develops branded generics, specialty, and OTC medicines, while prioritizing quality, regulatory compliance, and patient safety through continuous investment in digital quality and regulatory systems.
Prior to implementation, Glenmark faced operational and compliance challenges common to large, regulated pharmaceutical enterprises:
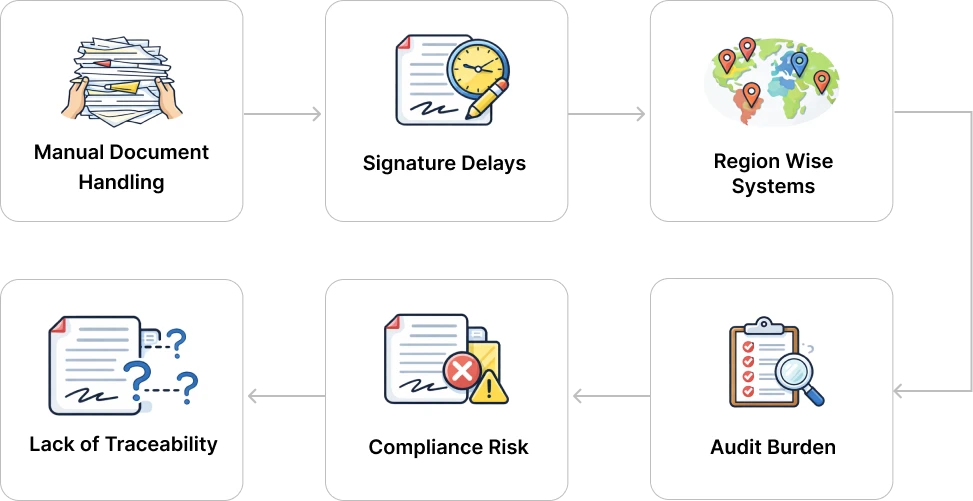
Glenmark implemented MSB Docs’ 21 CFR Part 11–compliant eSignature solution with API integrations to digitize and standardize document signing across its global operations.
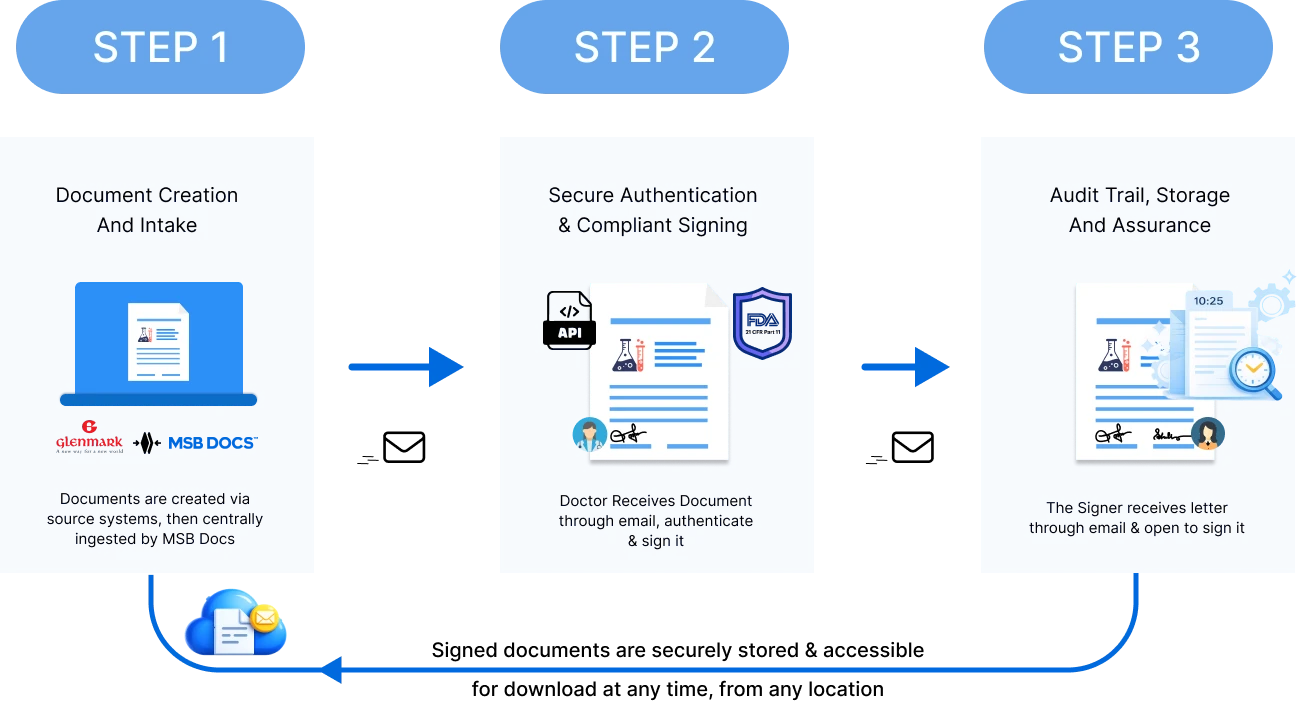
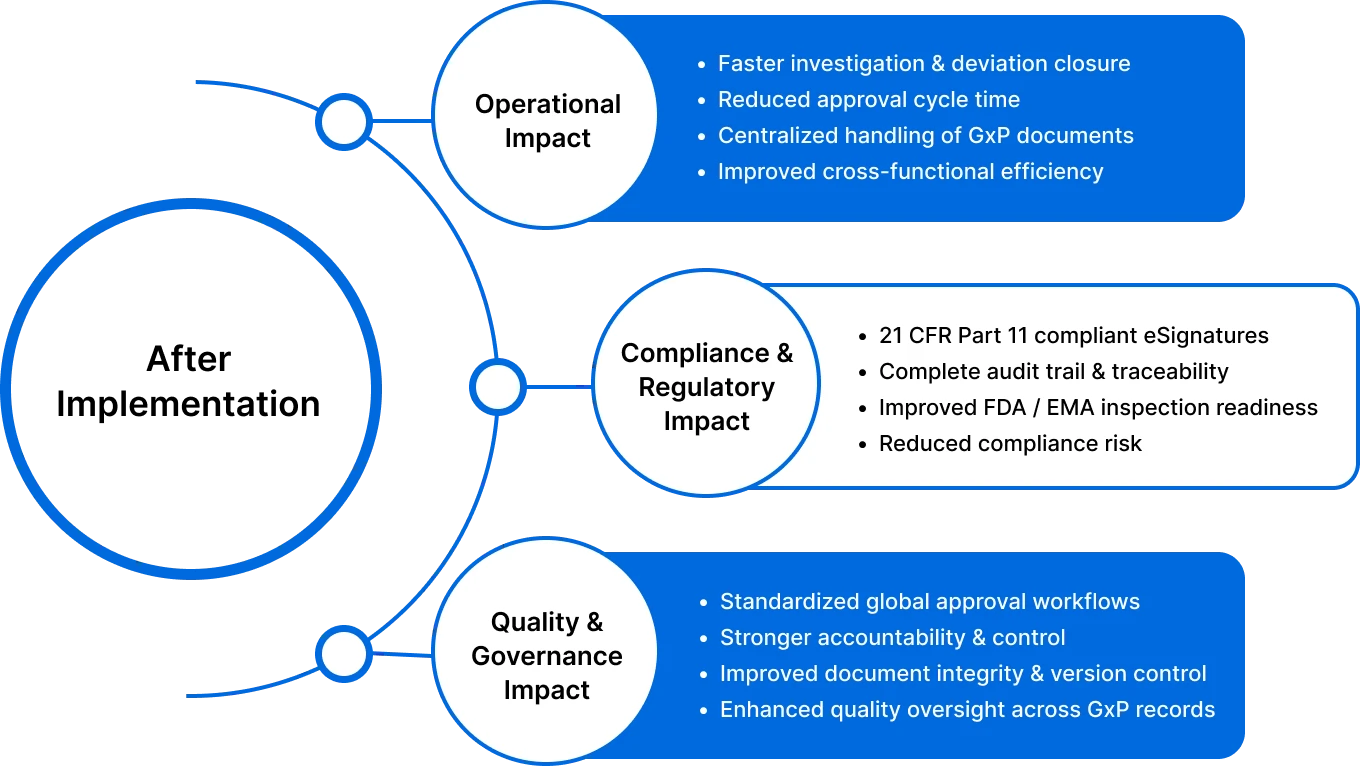
Glenmark’s digital solutions speed up approvals, improve collaboration, and ensure stronger compliance and quality control.
Document approvals
Time spent on audit preparation
Standardization of eSign workflows
By implementing MSB Docs’ 21 CFR Part 11 compliant eSignature solution with seamless API integrations, Glenmark successfully digitized and standardized its global documentation workflows. This transformation eliminated manual inefficiencies, strengthened compliance and governance, and enabled faster, audit-ready operations across all critical GxP processes.